
Aggravated Batteries Part I
Part I – Smoke Gets in Your Thighs
The surveillance camera video shows a man working behind a store counter when suddenly a shower of flames and smoke shoots from his pants pocket. It’s November of 2016 and the battery of the 31-year-old New Yorker’s e-cigarette device just exploded. He’s taken to a nearby hospital with burns requiring surgery to his leg and hand. Some e-cigarettes have exploded in users mouths, knocking out teeth and burning tongues. And it’s not just e-cigarettes. Also last year, Samsung recalled millions of new Galaxy Note 7 smartphones because of reports of overheating and fires.

Gives new meaning to a “phone blowing up.”
In addition to numerous reports of burn injuries, a Jeep was destroyed and a garage badly damaged (in separate incidents). When the replacement phones with “good” batteries started burning up as well, Samsung recalled the product and faced a three-billion-dollar loss. Laptops and tablets have also burst into flame. And remember the “hoverboard” scooters? So many fires were started by them that last July the Consumer Product Safety Commission forced the recall of half a million. Everywhere you turn, it seems like fiery electrical jinn are escaping their battery bottles to torment us. Why is this happening now, and what can be done to stop it? To address these questions, let’s first examine the nature and history of batteries.
The term battery dates to Benjamin Franklin in 1748. He used the term to describe an array of Leyden jars (an early type of capacitor) working together in analogy to a battery of artillery. The electrochemical batteries we think of today weren’t invented until more than half a century after that. In the 1780s, Luigi Galvani, a researcher in anatomy at the University of Bologna, formed a theory of “animal electricity” after running experiments making dissected frogs’ legs twitch [it’s alive!] by connecting the nerves and muscles with wires of different metals.
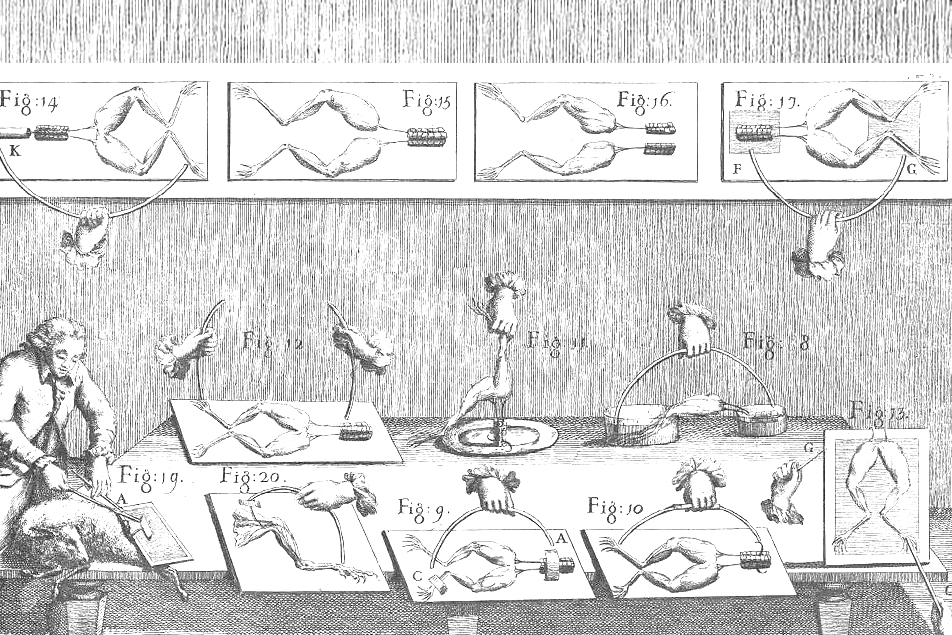
Quite a ribbiting experiment!
Although his theory was later disproven, its influence lives on. In 1816, Mary Shelley conceived the story of Victor Frankenstein in “a waking dream” following a day when the discussion had turned to galvanism.
In the interim, Alessandro Volta, a professor of physics at the University of Pavia, repeated Galvani’s experiments and came to a very different conclusion.
Realizing that the frog legs acted as both a conductor and detector of electricity, Volta replaced them with brine-soaked paper and detected the flow of electricity by other means. These results led, in 1800, to the construction of the voltaic pile – an early battery.
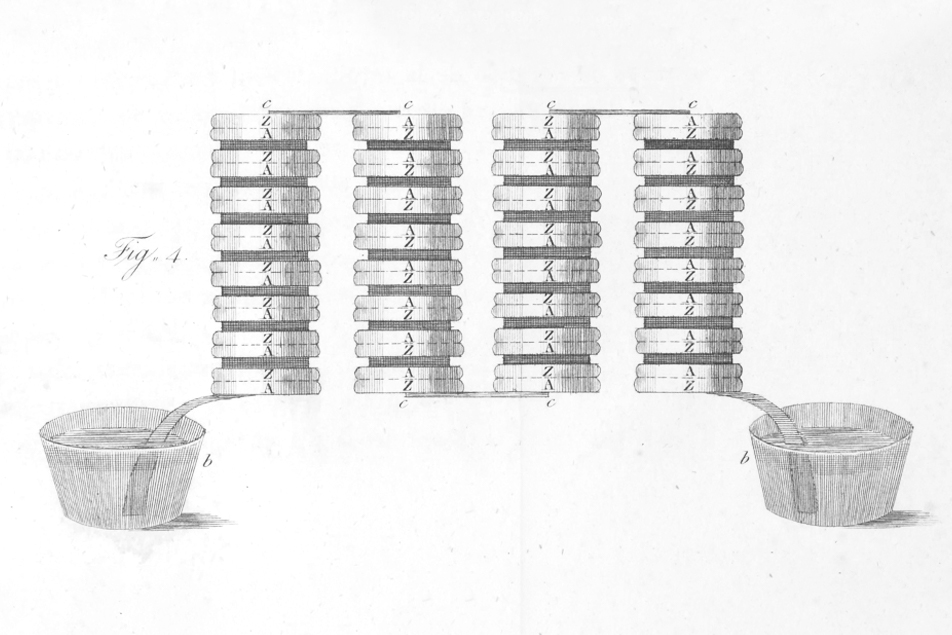
The Voltaic Pile (an early battery) by Alessandro Volta
The pile consisted of a stack of electrochemical cells, with each cell comprised of a disk of copper and a disk of zinc along with a piece of cardboard soaked in either diluted sulfuric acid or saltwater brine as a separator. This battery contained the basic elements of all batteries. The zinc metal dissociates into positive zinc ions and free electrons. Because it supplies electrons, it is the negative electrode, termed the anode. The electrons combine with positively charged hydrogen atoms (protons) from the sulfuric acid in the conducting solution, called the electrolyte, to form hydrogen gas. This actually breaks down the electrolyte in a process called electrolysis. In Volta’s battery, the copper does not react but simply acts as the positive electrode (cathode). By convention, electrical current flows from the cathode of the battery to the device being powered and then back to the anode. This is opposite to the flow of electrons (which weren’t discovered until much later).
For all you non-scientists out there that may have just glazed over the previous paragraph, we like this fun video explaining batteries.
Shall we continue?
These cells had problems. As the zinc dissolved in the reaction, small impurities in the zinc surface caused tiny short circuits to flow between adjacent regions, a phenomenon termed local action, which corroded the surface and caused the battery to degrade even when not connected. Hydrogen gas bubbles built up on surface of the copper disk, in a process termed polarization, increasing the internal resistance of the battery and, reducing the current. The sulfuric acid electrolyte was corrosive and dangerous when it leaked, and leakage between the disks also short-circuits the battery. The effect of these shortcomings was a useful battery life measured in a few hours at most, but as the first reliable source of constant current, it was a boon to research on electricity.
To this day, battery development continues to struggle to overcome similar problems.

Corrosion at its finest.
Despite the limitations, these early batteries provided a reliable source of relatively constant current for the laboratories of early electrical researchers.
They labored to improve the design of wet cell (liquid electrolyte and metallic electrode) batteries. The zinc corrosion was solved in 1835 by amalgamating it with mercury, and in 1836 the Daniell cell addressed the hydrogen build up by adding a separate electrolyte, copper sulfate solution, separated from the sulfuric acid solution surrounding the zinc electrode by an earthenware pot. The copper sulfate was contained in an outer copper pot which made the cathode. The earthenware prevented the copper ions from drifting to the zinc electrode when the cell was not connected. Instead of forming hydrogen gas by combining with the hydrogen ions, the electrons generated reduced the copper ions in the copper sulfate solution to copper metal. Unlike hydrogen bubbles, copper metal is a conductor, so the cells could operate for much longer, and the electrolytes were also less corrosive. Eventually, the pores in the earthenware would become filled with copper, preventing the ions from migrating. Still, the Daniell cell was the first practical source of electricity. Each cell provided 1.1 volts.
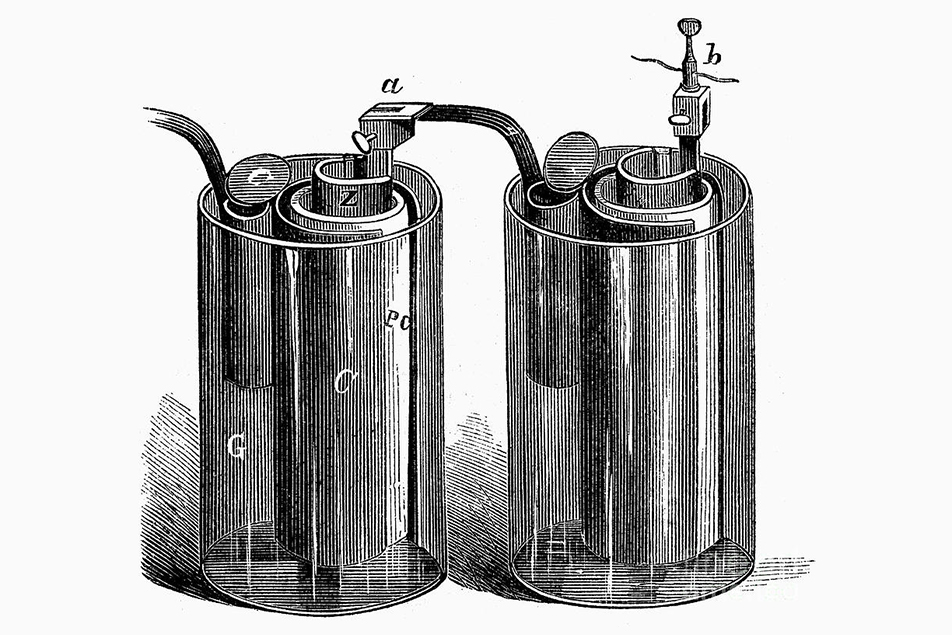
The Daniell cell – developed in 1836.
In the 1860s a modified form of the Daniell cell, called a gravity cell, was created. In it, the copper electrode sat at the bottom of a glass jar along with copper sulfate crystals. A zinc “crow’s foot” electrode was suspended near the top of the jar, which was then filled with distilled water. The crystals dissolved, forming copper sulfate solution. As current was drawn from the cell, a layer of zinc sulfate solution, which floated atop the higher density copper sulfate solution, formed around the zinc electrode and increased in thickness. There was no ceramic separator to be blocked by deposited copper, and the relative thicknesses of the blue copper electrolyte and clear zinc electrolyte made it easy to see how much charge was left. The cell could easily be renewed by replacing the exhausted copper electrolyte chemicals with fresh electrolytes. Because there was no separator, the cells had to remain stationary and current needed to be drawn continuously to keep the electrolytes separate. Still, its ease of use and maintenance quickly made it the choice for powering the British and American telegraph systems. Even after generators replaced batteries, these cells continued to power the local circuit in telegraph way stations into the 1950s, a remarkable hundred years of practical utility for this battery type.
Other wet cell designs used different electrolytes, electrode arrangements and separators. The Grove cell, an early contender for telegraphy applications, provided a higher current at nearly twice the voltage of the Daniell cell, but gave off toxic nitric oxide fumes and suffered sharp voltage drops as the charge diminished. All of these batteries were what is termed primary batteries; i.e. once the chemical reactants are consumed, the battery is dead. The liquid electrolytes also made them hard to use in mobile applications.
The first secondary (rechargeable) battery, the lead acid battery, was invented by Gaston Planté in 1859. It had a lead anode and lead dioxide cathode separated by rubber strips and immersed in sulfuric acid.
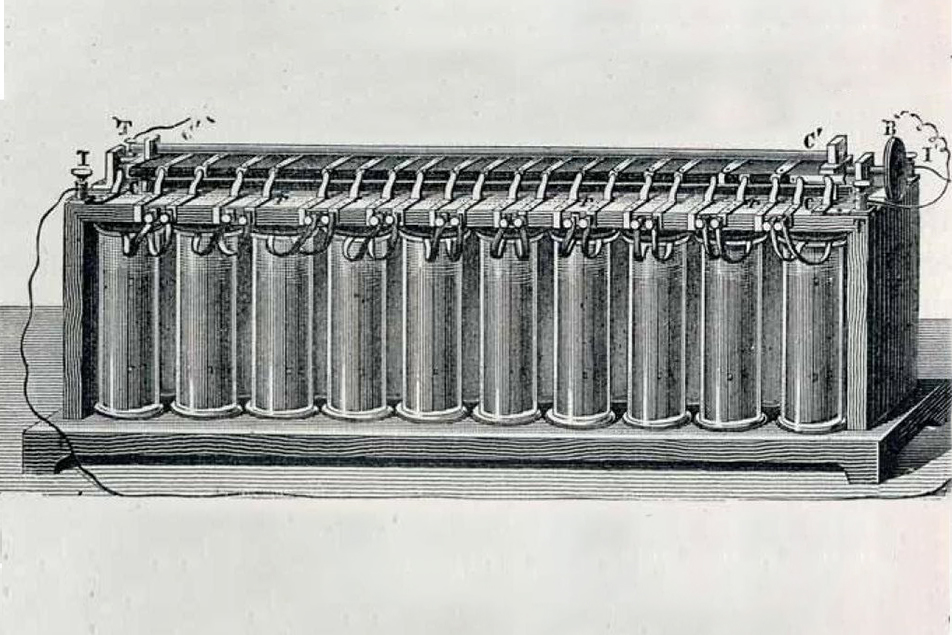
The first secondary battery invented by Gaston Planté in 1859.
In 1881, the design was improved by pressing lead oxide into a lead matrix to form a plate, with a cell potential of 2 volts. It’s a rugged design, which can operate in a wide temperature range, but has a low specific energy (energy to weight ratio). A big advantage is that it can deliver a high surge current, which is why this type of battery still finds use powering automobile starter motors as well as applications where weight is not a major consideration. Still, it has many of the same drawbacks as other designs. Water can evaporate from the electrolyte; so removable caps were needed to add replacement water. As chemistry students are cautioned, adding water to acid can generate a lot of heat, risking splashing with corrosive solution if not done carefully. The denser sulfuric acid can separate from the water, reducing performance, if it sits still too long (less a problem in vehicles). If left discharged too long, lead sulfate can form on electrodes, permanently damaging them. The crystals can even reach the separators, causing short circuits.
Overcharging electrolyzes the water into hydrogen and oxygen, which can build up to cause an explosion. Modern batteries have improvements which reduce these hazards.
One now-forgotten early use for lead-acid batteries was in early radios. The triode tubes in the early receivers were powered by three different batteries. The wet lead-acid “A” battery powered the tube filament, which required the most current. The “B” battery providing high voltage for the vacuum tube plate, and the “C” battery providing bias to the control grid, were dry batteries. During the 1920s, home AC electrical service started to be available and radios with rectifiers in place of batteries were produced, but power lines did not reach some U.S. rural areas until the 1940s.

Radioactive! (Get it?)
Some problems with wet cells were addressed by commercial production of zinc-carbon cell batteries in the late 1890s. In the modern version, a cylindrical outer zinc case forms the anode while a central carbon rod cathode, porous to allow gas escape, is topped with a metal cap. Adjacent to the outer case is a moist layer of ammonium or zinc chloride, while a layer of manganese dioxide and powdered carbon surrounds the cathode. Starch-coated paper separates the two layers. These 1.75v “dry cell” batteries did not require maintenance, couldn’t spill, and could be used in any orientation. The “D” cells quickly led to the development of hand-carried electric lights.
Early versions of the cells couldn’t provide a steady current to the inefficient carbon-filament bulbs, so they needed to be “rested” at short intervals to keep working, hence the nickname for the devices – flashlight.

The etymology of words is always enlightening.
One limitation of these batteries was a relatively short shelf life resulting from the acidic attack of ammonium chloride on the zinc shell, even when no current is drawn. Eating through the casing, ammonium chloride could leak out and corrode the contacts in the device. In 1939, a longtime maker of these batteries named Rayovac introduced “leak-proof sealed-in-steel” D cells, with a guarantee to replace your flashlight should leaking occur.
Zinc-carbon batteries powered the toys of the boomers, but their energy-to-weight ratio limited them to larger toys. Their service life of 110 minutes in continuous use necessitated frequent replacement, and a shelf life of 1-2 years meant stocking up was a bad idea. The development of alkaline batteries in the 1960s addressed these weaknesses.

Alkaline batteries were developed in the 1960s.
Although their external appearance is similar to zinc-iron batteries, the internal structure is quite different. The anode is a gel of zinc powder with alkaline potassium hydroxide surrounding an anode collector at the core of the cylinder. The cathode is layer of manganese dioxide and graphite adjacent to steel outer casing and separated from the anode by a layer of non-woven cellulose or synthetic polymer.
Although about 20% heavier than zinc-carbons, alkalines have 5 to 7 times the energy capacity and a shelf life as long as 5-6 years.
But they also have downsides. They’re more expensive than zinc-iron batteries, and internal resistance reduces their capacity in high-current applications like digital cameras. This can be improved by increasing the porosity of the anodes, making them out of cold pressed zinc wetted with mercury, but this increases the price as well as the hazardous waste problem. The biggest drawback is that they are prone to leaking the caustic potassium hydroxide solution. On exposure to air, the solution forms a growing feathery structure of potassium carbonate, which can spread from the contacts to device components, wicking the caustic solution, oxidizing copper electrical paths and resulting in permanent damage. The leakage guarantees of manufacturers were carefully modified (or eliminated) as a result. Alkalines also leak if they become dead with aging, which is why multiple batteries in a device should be replaced together.
Specially constructed alkalines can be recharged, but it is slow and capacity is significantly reduced with each cycle (usually you get no more than 20 useful cycles). Worse, recharging increases the likelihood of leaking. Still, these are the most popular general use primary batteries today.
For some applications, such as power tools, portable consumer devices, and uninterruptable power supplies, dry rechargeable (secondary) batteries are much to be preferred over primary batteries. We’ll look at the history of that class of batteries, and what’s causing our current spate of battery woes, in Part 2.