Aggravated Batteries Part II
Part II – Catching Lightning in a Bottle
Part 1 discussed the recent rash of battery fires in consumer products and reviewed the history of battery development, concentrating mainly on primary batteries – the kind you discard after discharging once. This part will discuss secondary (rechargeable) batteries, the causes behind the recent fires, and some of the research being done to develop better batteries for the future.
As described in Part 1, lead-acid batteries, like the one in your car, are rechargeable, can deliver large surge currents, and operate well over a wide temperature range, but have low specific energy (store relatively little energy for their weight). This is a real drawback for products like power tools or cellular telephones.
The need for rechargeable batteries which provide adequate power but weigh less drove the development of rechargeables based on different chemistries.

Fun fact: Lead-acid batteries (found in your car) are the most recycled consumer product in America.
For a practical rechargeable, the industry turned to nickel-cadmium (NiCd) batteries. Invented in 1899, they saw limited use until the 1950s, when sintered-plate construction was developed. The plates are made by compressing very fine particles of powdered nickel on a fine-mesh wire screen at high temperature. Positive plates (cathodes) are made by depositing nickel hydroxide on the plate, while cadmium hydroxide is deposited for negative plates (anodes). Tabs connect the sheets to their proper terminals. A porous plastic sheet is the separator, while a 30% solution of potassium hydroxide serves as an electrolyte. Cylindrical batteries are formed by rolling the plates in a “jelly roll” construction, so positive and negative layers alternate inside the nickel-plated steel case (which also serves as the anode).
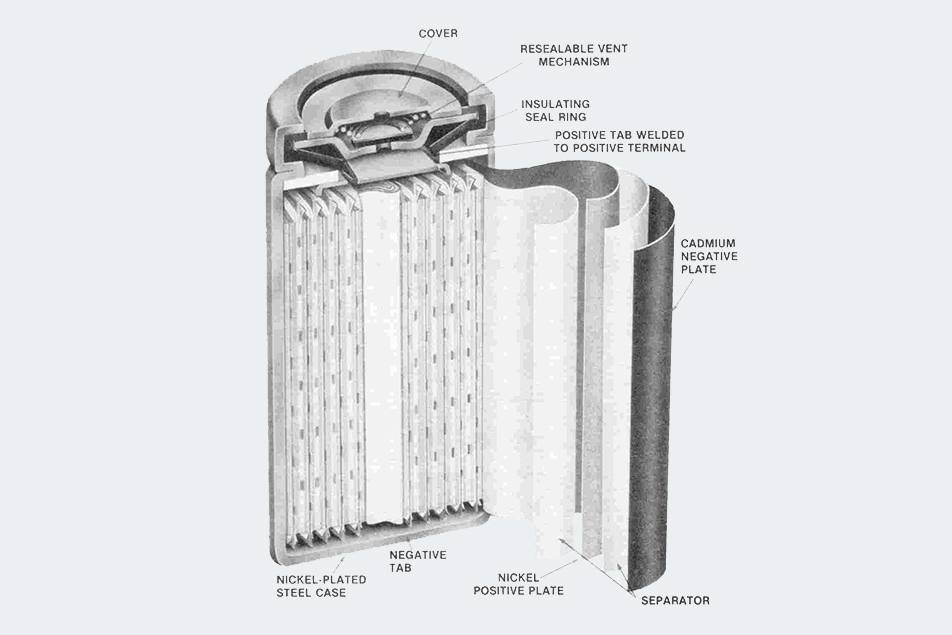
The Nickel-Cadmium (NiCd) battery was invented in 1899 but saw limited use until the 1950s, when sintered-plate construction was developed.
There is usually a valve safety vent to release hydrogen and oxygen from the sealed batteries if they build up excessive pressure during rapid charge or discharge. They have a cycle life 100 times greater than rechargeable alkaline batteries Nominal voltage is only 1.2 v, compared with 1.5 v for alkalines, but the voltage is very constant throughout discharge, while alkaline voltage drops linearly as it discharges. The energy per unit weight is only about 25% of alkaline batteries, but NiCds can operate at higher discharge rates than alkalines without sacrificing cycle life or capacity. However, they do suffer from what is called “memory” – if the battery is left in the charger for days at a time, or repeatedly discharged without a full recharge, the cadmium hydroxide recrystallizes to a larger size, reducing surface area and thus performance. In extreme cases, the crystals can pierce the separator causing a short. Hence, NiCds require “exercise” by doing a deep discharge every 1 to 3 months in order to get full performance and battery life. Charging itself is a bit tricky and can be done at different rates. A slow charge (16-24 hours) is recommended before use to bring all cells in the battery to the same charge since they self-discharge at different rates (averaging 10% per month – 30 times that of alkalines). Faster charging rates are allowed with most NiCds, but higher rates generate more heat, particularly close to fully charged when the temperature can rise to 120°F. Cheaper chargers use this temperature rise to monitor charging, but it is battery dependent, meaning it’s dangerous to use a different charger than the one supplied.
To mitigate NiCds limitations, hydrogen-absorbing alloys were introduced in the 1990s to replace the cadmium hydroxide. These Nickel-metal hydride (NiMH) batteries store about 75% of the energy of an alkaline battery and are superior to alkalines in high current applications like digital cameras. They display some memory effect, but it’s less than NiCds and is reversible.
Removing the toxic cadmium makes NiMHs recyclable, and the 2006 EU Battery Directive mandates they replace NiCds in portable consumer devices.
Starting with the replacement of vacuum tubes by transistors in the 1950s, electronics rapidly shrank in size and reduced in power requirements. Button cell batteries, with various chemistries, were introduced to power portable devices like watches, hearing aids and scientific instruments.
Button cells are often targeted for recycling because of the value of recoverable materials.
Though still used, they’ve declined in favor for several reasons. Rechargeable versions have no safety vent, so rapid charging can swell the cell and cause leakage, necessitating slow charging (10-16 hours). They often contain hazardous materials, complicating disposal and recycling. Worse, their small size invites ingestion by children, which can severely injure the esophagus.
Handheld cellular telephones were one of the chief drivers for improved rechargeable batteries. The Motorola DynaTAC 8000X, marketed in 1983, weighed 1.7 lbs. and cost the equivalent of $10,000.

In 1973 a prototype of what would evolve into the modern cell phone battery was first developed by an engineer at Bell Labs.
The Motorola DynaTAC 8000X used a NiCd battery that took 10 hours to charge and gave 30 minutes of talk time.
Through the 1990s, cell phones shrank in size and cost and increased in popularity. In 2000, around 50% of the developed world had a cell phone. Most of these were powered by a type of battery introduced commercially around 1990, the lithium-ion battery (Li-Ion). In these batteries, lithium ions migrate from the cathode to the anode during discharge. There are several types of Li-Ions with differing electrode compositions, but the type used in most portable devices, lithium cobalt oxide (LCO) has a cobalt oxide cathode and graphite anode. These materials are in the form of fine powders (to increase surface area) mixed with binders and coated in a thin layer onto a metallic current-collector foil. Lithium is very light, so LCOs have twice the specific energy (energy/weight) of NiMHs. They have nearly zero memory, so they are maintenance-free. Their self-discharge is also much slower than NiMH, although a protection circuit required to limit peak voltage introduces a small additional drain. At 4 volts, they have 3 times the nominal voltage of nickel rechargeables, so a single cell and can replace two or three NiMH cells in some applications for additional weight advantage. Charge time is the same as NiMH, and cycle life is about double (~500-1000 cycles). LCOs can be made into standard cylindrical battery sizes using “jelly roll” construction. They can also be made in a soft pouch form, usually encased for protection, which makes them useful for flat profile devices like smartphones and tablets.
Now, the battery could be designed to fit the device instead of designing the device to fit the battery.

Lithium ion battery packs and individual cells are equipped with protective circuitry variously monitoring temperature, voltage and charge to restrict charge and discharge rates to safe ranges.
All these advantages have made LCO the battery of choice for most portable and wearable electronic devices. Their high specific energy also makes them desirable for certain heavy-duty applications, like aviation and electric cars, where weight is important. Unfortunately, these advantages come at a price. Lithium is highly reactive with water, so the electrolyte is made of organic solvents containing lithium ions from dissolved lithium salts. The organic solvents have a larger increase in temperature than water when a given amount of heat is added. The reaction rates at the electrodes increase exponentially with temperature and the thin porous polyethylene/polypropylene film separators melt at 300°F. Battery packs and individual cells are equipped with protective circuitry variously monitoring temperature, voltage and charge to restrict charge and discharge rates to safe ranges. But should the protective circuitry fail and allow overcharge, or if there is short-circuiting due to improper connection or mechanical damage, and the temperature of the cell increases to around 175°F, a thermal runaway can occur in which the energy released further increases the temperature. With a fully charged cell, the temperature can reach in excess of 1100°F. Materials melt and internal pressure builds up from released gases, rupturing the cell and possibly ejecting the corrosive contents several meters.
The hot gases may ignite and, with the hot materials of the cell, start secondary fires or set off other cells in a battery. “Rapid disassembly” is the industry euphemism for this outcome.
One additional problem with LCOs is that they must be stored with a 40% to 50% charge because they are damaged if they dip below 2 volts for any length of time. Therefore, even before they are used, they contain large amounts of energy. In 2010, a shipment of lithium batteries aboard a UPS Boeing 747 flying in the UAE caught fire. Heat disabled the crew’s oxygen system and smoke completely filled the cockpit within 3 minutes. Both crew members were killed in the crash. In 2016 the UN aviation agency prohibited cargo shipments of Li-Ions on passenger planes.
Given that LCOs have been in use for decades, why has there recently been such a large number of prominent failures in popular consumer products? In part, it’s statistical. With an ever larger number of devices in use, even very low failure rates will result in a rising number of actual failures.
But it’s also the result of fierce competition to deliver more power, longer operating time per charge, and faster charging, with lighter batteries in thinner devices.
Current LCOs are at 90% of their theoretical capacity, so getting more power for brighter screens and faster processors, as well as the longest lasting charge, requires pushing the LCO design to its limit, increasing maximum charge voltage and reducing thickness of the separators. These changes increase the chance of failure.

Samsung’s notorious defects were aggravated by the use of an exceptionally thin separator to achieve high energy density needed.
In the case of the Samsung Galaxy Note 7, investigators found different manufacturing defects from the two companies which made the batteries. In the first set batteries, used for the initial rollout of the Note 7, the upper right corner of the battery was slightly missized causing it to be pinched in the device and causing shorts when the electrodes touched. There was a welding problem with the second set of batteries which didn’t become evident until production was rapidly ramped up to provide replacements for the first set. Both defects were aggravated by the use of an exceptionally thin separator to achieve high energy density needed as Samsung rushed to cram in new features and push the product to market ahead of Apple’s iPhone 7 (which itself has lately been plagued by fires).
What can be done? Additional protective circuitry probably won’t improve things much. The large lithium-ions, used in heavy-duty applications like electric cars, have multiple protections against thermal runaway built in. Nonetheless, Boeing’s new 787 Dreamliners were grounded for a time in 2013 due to problems with their lithium-ion batteries, including two thermal runaways.
One option is to use safer battery chemistries. Other lithium battery chemistries are less prone to thermal runaway. In particular, lithium iron phosphate batteries, which use that material as the cathode, are much safer. But, although they have a longer cycle life and higher current rating, their capacity (energy per weight) is about 60% of LCO’s. Lithium titanate, where that material replaces the graphite in the anode, are also much safer and charge faster than other LCB types, but their capacity is less than 50% LCO’s and they have a lower inherent voltage (2.4v). Lithium iron phosphate batteries already find use in applications like power tools and could be used for laptops as well if people would accept recharging more frequently.

Lithium iron phosphate batteries are safer but have a lower inherent voltage (2.4v); they are found in applications like power tools and could be used for laptops as well if people would accept recharging more frequently.
Longer term, bigger changes to chemistry may be able to deliver higher power, longer life, and faster charging along with increased safety. One possibility is aluminum-ion batteries, and several laboratories have tested different versions of these. While their theoretical voltage is lower than LCO’s, their theoretical energy density is more than twice LCO’s, but they are still far from production grade.
Another approach is to improve lithium-ion batteries by modifying the electrodes or electrolyte. For example, Stanford researchers mixed silicon nanoparticles with hydrogel to create electrodes which can hold much more lithium ions than graphite, and which retained their higher charge capacity through 6000 charging cycles. Tuft’s University Professor Mike Zimmerman is trying to improve safety by replacing the liquid electrolytes with a solid plastic electrolyte material. His prototypes can be cut and punctured with no combustion, and will even continue to deliver power as the battery is trimmed smaller and smaller with scissors.
However, it takes years to thoroughly test and scale up production of a new battery type.
Storing huge amounts of electrical energy in small volumes will always entail some amount of risk, so the ultimate solution for more power with greater safety might be to generate electricity as needed using micro fuel cells. Unfortunately, there are significant hurdles to overcome before they become feasible.
The legendary King Solomon, renowned for his wisdom, bound the fiery jinn using a magic ring called the Seal of Solomon.

The legendary King Solomon, renowned for his wisdom, bound the fiery jinn using a magic ring called the Seal of Solomon.
There is no magic ring for binding the power in our batteries, so we’ll have to rely on wisdom alone.
The wisdom of scientists to design better batteries. The wisdom of manufacturers to set realistic goals for power and performance of the devices they produce, and to test them appropriately. The wisdom of battery makers to push back against unrealistic requirements, and to improve their own test methods and manufacturing processes. And the wisdom of device users to handle with care, and treat with caution, the pent-up power which has been placed in their hands.